Lavender oil does not mimic estrogen nor does it enhance the body’s own estrogens. It is therefore not a ‘hormone disruptor’, cannot cause breast growth in young boys (or girls of any age), and is safe to use by anyone at risk for estrogen-dependent cancer. The lack of estrogenic action is the conclusion of a new report, which used a novel form of ‘uterotrophic’ assay.
This measures the effect of a test substance on the uterus of immature or estrogen-deprived female rats over three days. Any estrogenic action causes a rapid and measurable increase in uterine weight. The assay has been in use since the 1930s, was adopted by the OECD in 2007, and is now regarded as the “benchmark animal assay for estrogenic effects” (Politano 2013).
A 2007 report by Henley et al found that both lavender and tea tree oils had a weak in vitro estrogenic action. Lavender was suggested as the cause of three cases of prepubertal gynecomastia (breast growth) in boys, and tea tree was suggested in one case. The report was subsequently criticized on a number of grounds. For example it was pointed out that there was no evidence of either essential oil causing the gynecomastia in any of the four cases, and that in vitro estrogenic findings frequently do not extrapolate to a similar action in warm bodies (see Rebuttals, below).
Since 2007 there have been many warnings about lavender and tea tree oils that relate back to this study. Others, including myself, have suggested that such cautions are unnecessary and premature. The new research, in which considerable quantities of lavender oil were used, found that there was no increase in uterine weight, and so no estrogenic action.
The novel aspect of the uterotrophic assay was that the test substance – lavender oil – was applied to the skin, while the most common method is subcutaneous injection. This was changed in order to mimic the circumstances of the Henley et al 2007 case reports, and it also mimics the use of lavender oil in fragrances and personal care products. Lavender oil was used in two concentrations, 4% and 20% in corn oil. According to Politano et al 2013, these concentrations are respectively more than 6,000 and 30,000 times greater than a conservative estimate of human skin exposure from multiple cosmetic products containing lavender oil. They are also 5,000 and 1,000,000 times greater than the estimated exposure to lavender oil experienced by the Henley et al boys.
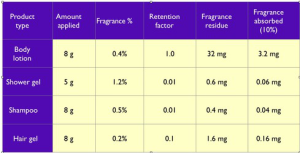
These calculations may seem exaggerated, but the massive differences are because in the uterotrophic assay, the lavender oil was applied in Hilltop Chambers patches, which do not allow any evaporation, or loss of essential oil by means other than dermal absorption. Examples of the quantities of fragrant substance absorbed from personal care products are shown below. The amount of fragrance absorbed from a shampoo, for instance, is 200 times less than the amount applied to the head. If amounts of dermally-absorbed lavender oil that are at least 5,000 times greater than any normal human exposure are not estrogenic, then we can be confident that this particular safety issue is not a concern.
Safety in pregnancy is a broader question than hormonal action alone, since affects on the fetus, or even miscarriage, are theoretically possible with any substance. The question of uterine stimulation and miscarriage was thoroughly investigated here, and there is clearly no risk. As for fetal effects we know that linalool, a major constituent of lavender oil, is not fetotoxic to pregnant rats at an oral dose 1 g/kg/day for 16 days (equivalent to a human oral dose of 60 g, or 2 oz per day, and a total dose of 32 oz). The chances of lavender oil being fetotoxic in the amounts used in personal care products are negligible, considering the small amounts both applied to, and subsequently absorbed by human skin (Cadby et al 2002).
The new research findings represent a major development in our knowledge of lavender oil safety, since the possibility of estrogenic action now looks remote. While no single test should be taken as absolutely conclusive, we can expect the volume of noise about lavender being estrogenic to diminish considerably.
Rebuttals
Dean CJ 2007 Prepubertal gynecomastia linked to lavender and tea tree oils. New England Journal of Medicine 356:2543-2544
Kalyan S 2007 Prepubertal gynecomastia linked to lavender and tea tree oils. New England Journal of Medicine 356:2542-2544
Kemper KJ, Romm AJ, Gardiner P 2007 Prepubertal gynecomastia linked to lavender and tea tree oils. New England Journal of Medicine 356:2541-2542
Kurtz JL 2007 Prepubertal gynecomastia linked to lavender and tea tree oils. New England Journal of Medicine 356:2542-2543
Lawrence BM 2007 Estrogenic activity in lavender and tea tree oils: Part I. Perfumer & Flavorist 32:20-25
Lawrence BM 2007 Estrogenic activity in lavender and tea tree oils: Part II. Perfumer Flavorist 32:14-20
Tisserand R Tea tree and lavender not linked to gynecomastia
Other references
Cadby P A, Troy W R, Vey M G 2002 Consumer exposure to fragrance ingredients: providing estimates for safety evaluation. Regulatory Toxicology & Pharmacology 36: 246-252
Henley DV, Lipson N, Korach KS, Bloch CA 2007 Prebubertal gynecomastia linked to lavender and tea tree oils. New England Journal of Medicine 365(5): 479-485
Politano VT, Lewis EM, Hoberman AM et al 2008 Evaluation of the developmental toxicity of linalool in rats. International Journal of Toxicology 27:183-188
Politano VT, McGinty D, Lewis EM et al 2013 Uterotrophic assay of percutaneous lavender oil in immature female rats. International Journal of Toxicology



I really appreciate your research and these informative articles. I remember attending a workshop in Miami a few years ago and you covered the myth about lavender and breast growth for young boys. It makes me feel more at ease when using essential oils knowing that there is plenty of research supporting the safety of its use.
Thank you
Yolanda
Thanks for posting this Robert; as usual a well researched article rebutting the myth that lavender is estrogenic.
The work of Politano et al is both elegant and conclusive; we have asked them to repeat the work with pure Australian tea tree oil which, like lavender, suffered incalculable harm through media hype when Henley et al published their paper linking these essential oils to gynecomastia.
Tony Larkman
Australian Tea Tree Oil Industry Association (ATTIA Ltd)
As always, thank you for ‘clearing the air’….it is amazing how many people believe that lavender is “dangerous” based on that misguided publication!
Thanks Robert, I had heard of the potential oestrogenic effects of these oils but was inclined to not trust it. Not directly relevant to any of my clients since I heard of it but it may well be relevant for future clients. Good to know that my instincts are fight on this one.
The assay will detect direct estrogenic effects but would not be able to detect the potential to stimulate the release of estrogen in the body. The initial report suggested a link and it may be flawed but the above mentioned doesn’t completely vindicate Lavender oil.
Hi Don, I’m not sure why you say that the uterotrophic assay could not detect the potential to stimulate the release of estrogen. Surely it picks up on any estrogenic action, direct or indirect. I agree, the report does not completely vindicate lavender oil (which I do say in my post) but it makes the probability of an estrogenic action extremely remote.
I’m curious then, why is lavender reported to be in some breast enlargement pills? Anything that causes breast enlargement would be affecting hormones in some way, correct? I had estrogen positive breast cancer and I have been cautious to avoid lavender, so I am interested in this new study.
Hi kel, I guess the issue is – do these breast enlargement pills work? And if so, which are the active ingredients? There are several breast enlargement creams based on essential oils that are sold in China, but they don’t do anything. But yes, you would expect such as effect to be hormonal. As for lavender oil, it’s not going to have a hormonal effect, so not at all likely to affect breast volume.
Thanks for posting this! Extremely helpful concerning the use of Lavender and the claims of it having the estrogenic and antiandrogenic activities.
This is good to know and comforting, although I did notice that they only described the use of it at a 5% and 20% dilution rate!! And only topically (or dermal use).. I would like to see the results of this study using lavender oil Neat and also possible affects of it.. diffused … Where the molecule droplets of oil actually enter the very vascular nasal cavity…ya know!
Here’s my beef. As consumers we should question any and all study’s regardless the subject. Unless we have pertinent information such as ; a) who FINANCED the study, b) the name of the Research facility that generated the findings, c) who are the players in this “pro vs con” debate and are either vested financially in an outcome ? Thank You
Hi Robert
Thanks for clearing this up and your research is well informed and carried out. As a coordinator for a charity that provides complementary therapies for people who have been diagnosed with cancer, it is a relief to come over your research I thought I may have to withdraw all our lavender and tea tree essential oils! The charity is called ‘The Lavender Touch’ Its unfortunate that the information surrounding the so called estrogenic effects of these oils are still circulating in classes that are supposed to be training trained aromatherapists and massage therapists in cancer care. Thank you for your work
Thanks for bringing this to light.
If it was a prescription drug that possibly increased breast tissue in a couple of boys it would never have made it into a medical journal. It has been a while since I read the article on this but if I remember right no one questioned the possibility of the type of plastic that the lavender product was in thus the possible exposure to phthalates, or BPA that are known endocrine disruptors.
Marijuana use is also a potential cause of gynecomastia. Not every adolescent or preadolescent boy is going to volunteer that he has been using marijuana.
Question: is it fair to infer results related to gynecomastia when the rebuttal studies were done on female mice and the effect on their uterus? We’re talking boys, how can results be one and the same? Shouldn’t they have been using pre-teen (equivalent) male mice and examining their breasts?
The results of the uterotrophic assay are relevant, because they measure estrogenic action (an increase in estrogen) and this is also the cause of gynecomastia in young boys. However, it has never been established that the boys’ gynecomastia was caused by essential oils, and this is now very much in doubt. It is now believed that the most likely cause of the in vitro estrogenic effect seen for lavender oil and tea tree oil was due to phthalates (which are estrogenic) being leached out of the plastic labware used in these tests. We know that essential oils can and do leach such compounds out of plastics https://roberttisserand.com/2014/02/estrogens-in-plastic-labware/