Safety legislation does not always accord with current knowledge on safety, for the simple reason that new scientific data are always being published. Guidelines are periodically made more stringent, but they are almost never loosened, even when new information suggests that this would make sense. Regulators don’t like to admit that they were wrong, and this is especially true of the European Union. In the United States, although the FDA has few regulations that directly restrict cosmetic ingredients, most manufacturers, especially the larger ones, follow both IFRA guidelines and EU regulations. Taken together, these result in some extremely stringent measures for essential oils.
The reason that US manufacturers follow EU guidelines is because, if they sell internationally, they use one formulation that works in all regions – multiple formulations are uneconomic. And, although IFRA guidelines are technically a voluntary code, they are very widely adhered to for two reasons. One, most suppliers of fragrance to the large cosmetic companies are full members of IFRA, and as such they formally agree to follow the IFRA code. Two, even non-members want to be sure they are manufacturing safe products, plus they don’t want to risk the possible legal ramifications of not adhering to industry best-practice guidelines. IFRA recently put out a video called Making Scents, which you can find here.
Coriandrum sativum
In spite of all this, some North American consumer groups are concerned that many personal care products contain ingredients that are highly toxic, and that are banned in Europe. There are particular concerns about fragrances, which are said to contain chemicals that are hormone disrupting, neurotoxic, teratogenic or carcinogenic. The fact that fragrance ingredients are not declared on labels feeds the perception of hidden toxins lurking. However, these concerns are often misplaced. For example, fears of neurotoxicity may be inappropriately based on the results of toxicity testing, in which the signs and symptoms of a fatal dose are noted. And, concerns about skin allergy are sometimes based on results that, when closely examined, do not represent a significant risk for consumer products.
There is a growing hysteria about “chemicals” in consumer products, as if the fact of a substance being a chemical made it inherently toxic. It is understandable that consumers do not know the difference between a synthetic chemical and a naturally-occurring one. (Synthetic chemicals, while not necessarily more toxic, are less environmentally friendly.) However, even the Environmental Working Group appears not to know which essential oils contain which chemical constituents.
The European Union “allergens”
In 2003, the European Union’s Scientific Committee on Cosmetic Products and Non-Food Products (SCCNFP) published a directive listing 26 fragrance materials as skin allergens (SCCNFP 1999). One of the criteria listed was that “Positive patch test data from more than one patient in more than one independent centre should be present.” In other words, a substance could be listed as an allergen if there were two or more reports of skin allergy. Even if these two reports occurred over, say, 20 years. Several papers have since been published strongly suggesting that many of the 26 fragrance materials should not be listed as allergens at all. The EU has done nothing but dig its heels in.
Linalool is one of the EU “allergens”. If present in a cosmetic product at over 100 ppm (0.01%) in a wash-off product or 10 ppm (0.001%) in a leave-on product, linalool must be declared on the ingredient list if sold in an EU member state. Doesn’t sound too bad, does it? The problem is, neither manufacturers nor retailers want to get sued, or branded as selling unsafe products, and most retailers will only carry cosmetics that have passed an independent safety assessment, which is almost entirely based on looking at the levels of “allergens”. So the de facto result is that very few manufacturers take the risk of having a “known allergen” in a product at over the declarable amount.
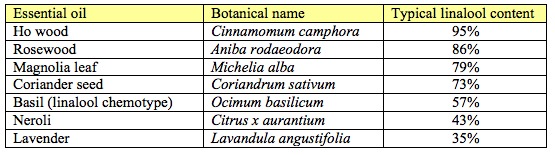
Linalool is a major constituent of some commonly-used essential oils and is found in approximately 200 other essential oils. But linalool is not a high-risk allergen. In fact, it’s superlatively safe on the skin. Between 1969 and 2007 (38 years), a total of thirteen dermatitis patients out of the 25,164 tested, (0.05%) were allergic to linalool when patch tested, and less than this actually had allergic reactions to products containing linalool (De Groot 1987, De Groot et al 2000, Fregert and Hjorth 1969, Frosch et al 1995, Itoh et al 1986, Santucci et al 1987, Schnuch et al 2007). Yes, 0.05% is more than zero, but it’s pretty close to the 0.03% reaction rate for petrolatum, the least dermally allergenic substance known to mankind. One way of looking at this is that adding linalool to a product increases risk by about 0.02%. That’s probably less than almost any other known cosmetic ingredient.
 But, this assumes that patch testing reflects real-world risk, which it does not, in fact it is designed to exaggerate risk. It does this in two ways. One, patches are non-permeable, and are left adhered to the skin for 48 hours. Two, the concentrations used in testing are higher than those encountered in personal care products. Linalool is tested at a 5%, 10% or 20% dilution. Since skin allergies are dilution-dependent, lower dilution will carry less risk. There is no dermatological or other scientific rationale that suggests extrapolating data from a 10% dilution to a safety threshold of 0.001% – 10,000 times less! Quite the opposite – the clinical data suggest that a 10% concentration of linalool in cosmetics is virtually non-allergenic. When tested at 5% on a total of 1,399 dermatology patients, linalool produced not one single allergic reaction (Frosch et al 1995, Itoh et al 1986, Santucci et al 1987).
But, this assumes that patch testing reflects real-world risk, which it does not, in fact it is designed to exaggerate risk. It does this in two ways. One, patches are non-permeable, and are left adhered to the skin for 48 hours. Two, the concentrations used in testing are higher than those encountered in personal care products. Linalool is tested at a 5%, 10% or 20% dilution. Since skin allergies are dilution-dependent, lower dilution will carry less risk. There is no dermatological or other scientific rationale that suggests extrapolating data from a 10% dilution to a safety threshold of 0.001% – 10,000 times less! Quite the opposite – the clinical data suggest that a 10% concentration of linalool in cosmetics is virtually non-allergenic. When tested at 5% on a total of 1,399 dermatology patients, linalool produced not one single allergic reaction (Frosch et al 1995, Itoh et al 1986, Santucci et al 1987).
The EU listed linalool as an allergen because – according to their own report – five dermatitis patients had allergic reactions to it over a five-year period on patch testing. Considering that linalool is (or at least used to be) one of the most commonly-used fragrance materials, an average of one reported adverse reaction per year, on planet earth, is about a negligible as it is possible to get. But, this still does not represent actual risk to consumers, which is likely much lower.
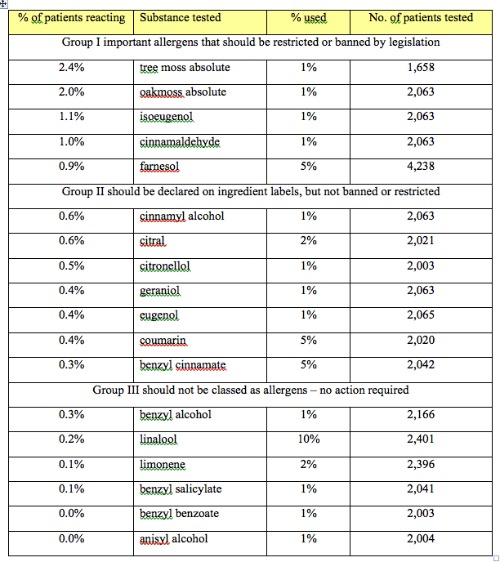
Data from Schnuch et al 2007
Of the 26 EU “allergens”, 16 are essential oil constituents and two are absolutes. In 2007, these were each tested on groups of 2,000 or more dermatology patients. Of the 16, six produced so few adverse reactions that the report concluded that they should not be classed as allergens at all. Benzyl benzoate, for example, produced not a single adverse reaction in 2,003 patients (Schnuch et al 2007). The other non-allergenic constituents are linalool, limonene, benzyl alcohol, benzyl salicylate and anisyl alcohol, and other dermatologists have questioned the classification of linalool and anisyl alcohol as allergens (Gilpin and Maibach 2010, Hostýnek and Maibach 2003a). Other research has shown that adverse reactions to coumarin are due to impurities present in the synthetic coumarin used for testing, and that 99% pure coumarin is not allergenic (Vocanson et al 2006, 2007). And, Hostýnek and Maibach (2003b) argue that the evidence for farnesol being an allergen is highly debatable. If we add farnesol and coumarin to the list of spurious allergens, then 50% of the EU 16 are a mistake.
These voices of dissent are not insignificant, and include some of the most distinguished dermatologists in the world. They question whether the patch test information is “clinically relevant”, and whether it can be extrapolated to estimate risk in the general population. Certainly, the percentages in the Table above under “% of patients reacting” do not represent real-world risk, and for many of these substances there is not a single case of skin reaction that has been proven to be caused by the substance in question. What these numbers do suggest is the relative potency between the different substances. Or at least, it would if they had all been tested at the same % concentration. And just to be clear, the division into three groups by Schnuch et al is theirs, not mine.
The David Suzuki Foundation
Paradoxically, EU cosmetics legislation is frequently cited in North America as an example of what cosmetics legislation should look like. In Canada for example, the David Suzuki Foundation (DSF), an environmental activist group, has this message for their supporters: “Consumers have the right to know about all ingredients contained in cosmetics – including fragrance chemicals. European regulations are stronger. They require 26 sensitizers used as cosmetic fragrances to be identified on the label. That’s a start, and it’s better than what we have in Canada.”
The DSF says that their mission is “to protect the diversity of nature” but the European legislation unfairly targets the farmers that grow the plants that produce the essential oils that contain the chemicals that David Suzuki wants to see identified on labels, a move which will inevitably lead to further restriction. I am not opposed to the principal of ingredient declaration for fragrances, and I applaud those manufacturers that have already made this move. However, I believe that if a product contains lavender oil, this should be declared as “lavender oil”, and the 70 or so constituents of lavender oil should not have to be listed. I have already argued here against the idea that constituents of ingredients should be declared on cosmetic labels.
The Environmental Working Group
The Environmental Working Group (EWG) is a US-based organization that calls even more stridently for increased legislation of fragrance ingredients. Fragrances, we are told, contain chemicals that are neurotoxic, teratogenic, carcinogenic and hormone disrupting.
 On its Skin Deep database, the EWG bases hazard ratings of essential oil constituents largely on the flawed EU legislation. The EWG makes no reference to the dissenting voices in the scientific community, either because it is unaware of such dissent, or because it chooses to ignore it. The EWG is not a regulatory body, nor does it publish safety guidelines, it simply labels a cosmetic ingredient with a number from 0 to 10, with 10 being the most hazardous. It does give some explanation for how this number is arrived at, but no specific recommendations are made. Skin Deep gives linalool a hazard rating of 4. However, Aniba rosaeodora (Rosewood) oil, which contains 82-90% linalool, has a hazard rating of 0-1. Coriander seed oil, which contains 59-88% linalool, has a hazard rating of 1. These hazard ratings seem to be inconsistent.
On its Skin Deep database, the EWG bases hazard ratings of essential oil constituents largely on the flawed EU legislation. The EWG makes no reference to the dissenting voices in the scientific community, either because it is unaware of such dissent, or because it chooses to ignore it. The EWG is not a regulatory body, nor does it publish safety guidelines, it simply labels a cosmetic ingredient with a number from 0 to 10, with 10 being the most hazardous. It does give some explanation for how this number is arrived at, but no specific recommendations are made. Skin Deep gives linalool a hazard rating of 4. However, Aniba rosaeodora (Rosewood) oil, which contains 82-90% linalool, has a hazard rating of 0-1. Coriander seed oil, which contains 59-88% linalool, has a hazard rating of 1. These hazard ratings seem to be inconsistent.
Skin Deep, at least, is consistent in its inconsistency. Limonene has a hazard rating of 6, and yet lemon oil (57-76% limonene) has a hazard rating of 0, and sweet orange oil (84-96% limonene) a hazard rating of 1. Safrole (a rodent carcinogen) is given a hazard rating of 7, while sassafras oil (83-90% safrole) is given a hazard rating of 0. Sassafras oil contains more safrole than any other essential oil. Some other carcinogens found in essential oils, asarone and estragole for instance, are not even mentioned on the Skin Deep database. Pulegone is a hepatotoxic compound found in pennyroyal oil. In spite of this, both the compound and the essential oil are rated as 0. Go figure.
[September 2016 update on the above info: limonene is still 6; there are two entries for lemon oil, one is a 1, the other a 3; orange oil is still 1; safrole and pulegone are no longer mentioned at all; estragole (methyl chavicol) and asarone are still absent; sassafras oil (which by the way cannot be legally sold) is now rated 4; pennyroyal oil is now rated 1.]
Fragrance
If you look at “Fragrance” on the EWG’s Skin Deep database, you will see that it has a rating of 8. This applies to any fragrance at all, and 11,376 products are listed. This seems more like a declaration of war on the personal care products industry than a genuine safety guideline! And note that “fragrance” is rated as far more hazardous than either sassafras oil (a known carcinogen) or pennyroyal oil (a known hepatotoxin). The principal reasons given for the high rating for fragrance are:
Allergies & immunotoxicity
Miscellaneous
Neurotoxicity
Data gaps
It’s worth taking a closer look at the Skin Deep rationale:
Allergies & immunotoxicity
This is further defined as “linked to immunotoxicity, or harm to the immune system, a class of health problems that manifest as allergic reactions or an impaired capacity to fight disease and repair damaged tissues in the body.” Perfume is then cited as a “known human immune system toxicant”, and a single reference is given: SCCNFP 1999. This is the opinion paper that eventually became a legal directive in 2003.
Since this is a 63 page document, there is insufficient space here to dissect it in detail. To pick one simple fact, the document concerns 24 fragrance ingredients that, it is recommended, should be restricted in consumer products because they are potential contact allergens (oakmoss absolute and treemoss absolute were added later). This is to say, 24 of the estimated 3,000 existing fragrance ingredients, or 0.8%. To conclude from this that all fragrances present a high, or even a moderate risk of skin allergy is negative bias, because it is not based on real-world risk.
Returning to the Skin Deep wording, something is amiss. A single reference is given for skin allergy, but no supporting evidence is cited for immunotoxicity, which is a much more serious hazard. This could be viewed as a deliberate manipulation of words and/or facts in order to mislead and suggest negative information that does not exist. Skin allergy is indeed a sub-category of immunotoxicity, but the principal meaning of the word – causing damage to the immune system – does not apply. But, because Skin Deep couches these terms together “Allergy/Immunotoxicity”, and because it has – quite correctly – defined immunotoxicity as damage to the immune system, any substance that can cause skin allergy is also flagged by implication, as reducing your capacity to fight disease, which is something totally different. Since there is no evidence of immunotoxicity, apart from skin allergy, this looks like negative bias again.
 Miscellaneous
Miscellaneous
This is defined as “ingredient not fully labeled – identity unknown”. Indeed, fragrance is not a single ingredient, and the great majority of fragranced products do not fully declare their fragrant ingredients. This has been a subject of debate for some time, and is a reasonable criticism in terms of transparency. However, it is not, per se, any kind of risk assessment or toxicity rating, it is simply a fact, an observation.
Neurotoxicity
This is defined as “Linked to neurotoxicity, or harm to the brain and nervous system, a class of health problems that can range from subtle developmental delays to chronic nerve degeneration diseases.” One reference is given, which is said to provide “moderate evidence” of neurotoxicity. The reference is: USHR (U.S. House of Representatives), 1986. Neurotoxins: At Home and the Workplace. Report by the Committee on Science & Technology, Report 99-827. Sept 16 1986. In this report it is claimed that over 95% of chemicals used in fragrances are synthetic compounds derived from petroleum, including benzene derivatives, aldehydes and other toxins and sensitizers capable of causing cancer, birth defects central nervous system disorders and allergic reactions.
The report is not a scientific study, and so what we have is nothing but hearsay. Somebody said/wrote something, so the “has been linked to” is satisfied! All fragrances have now “been linked to” neurotoxicity. This is a very serious charge. Note that the EWG claim is that they “provide additional information on personal care product ingredients from the published scientific literature.” Not always it seems. And note that ALL FRAGRANCE is flagged as being “linked to” neurotoxicity. “Benzene derivatives, aldehydes and other toxins and sensitizers” is, by the way, an interesting choice of words in itself, since it implies that all the benzene derivatives and/or aldehydes used in fragrances are toxic and/or skin sensitizing. This is simply not true.
Data gaps
This is explained as “not assessed for safety in cosmetics by industry panel.” This cryptic statement is odd to say the least. The implication is that no fragrance-related organization has assessed “fragrance” for safety in cosmetics. It seems that Skin Deep are unfamiliar with an organization called IFRA – the International Fragrance Association – that has been assessing fragrance for safety in consumer products for some 40 years. IFRA has many fragrance-related safety standards. That’s pretty much all they do. In my opinion, IFRA standards are often over-reaching and too stringent. So, what exactly is meant by “Data gaps” for fragrance is, well, anyone’s guess.
At the end of the Skin Deep page on Fragrance is some useful information: “1,452 studies in PubMed science library may include information on the toxicity of this chemical” And then there is a link to PubMed. These are the search criteria: (“FRAGRANCE”[TW] OR “FRAGRANCE”[TW] OR “PARFUM”[TW] ) AND (*toxic* OR cosmet* OR derm* OR irritation OR sensiti* OR “personal care products” OR skin OR gavage OR mutagen* OR carcinogen* OR “biological activity”). Fine, great, useful, practical. What I really don’t get though, is why these 1,452 research papers are listed under the heading “Data gaps”. Isn’t this actually quite a lot of information?
Perhaps the Skin Deep approach is: “if you won’t tell us what’s in your fragrances, then we’re going to assume the worst”. But, since there’s very little evidence that fragrance causes any real harm anyway, assuming the worst involves some academic acrobatics that are shameful and not worthy of scientific credibility. Insinuation, implication and “has been linked to” is not evidence of anything, and the liberal use of this tactic shows negative bias.
Linalool: a narcotic?
A Google search for “Linalool: a narcotic” comes up with 2,900 hits. This is partly because the following piece of advice about a well-known fabric softener and dryer sheet fragrance is repeated so many times:
* Ethanol: On the EPA’s Hazardous Waste list and can cause central nervous system disorders.
* Limonene: Suspected Gastrointestinal or Liver Toxicant, Immunotoxicant, Kidney Toxicant, Neurotoxicant, Respiratory Toxicant, and Skin or Sense Organ Toxicant.
* A-Terpineol: Can cause respiratory problems, including fatal edema, and central nervous system damage.
* Ethyl Acetate: A narcotic on the EPA’s Hazardous Waste list.
* Camphor: Causes central nervous system disorders.
* Chloroform: Neurotoxic, anesthetic and carcinogenic.
* Linalool: A narcotic that causes central nervous system disorders.
I’m not going to go into the validity of every single claim made here, but I will tell you that most of it is either incorrect or highly misleading. Ethanol for example, known to most of us simply as alcohol, can of course cause CNS disorders if you drink enough of it. But in a dryer sheet? Are you kidding? Some of the sites that include the above information go into more detail on linalool:
LINALOOL Narcotic. Causes CNS disorders. …”respiratory disturbances” …”Attracts bees.” “In animal tests: ataxic gait, reduced spontaneousmotor activity and depression …depressed heart activity …development of respiratory disturbances leading to death.”
 This information is entirely derived from LD50 testing of linalool (Jenner et al 1964, Letizia et al 2003). This is the classic test to find the single lethal dose for any substance. Rats and mice are most commonly used, and the dose cited is the one that is lethal to 50% of the animals. When you give a mammal a fatal dose of a substance it is not unusual to see some adverse effects on the nervous system, such as staggering, difficulty breathing etc., nor is it surprising if there are “respiratory disturbances leading to death.” Ataxic (unsteady) gait is probably mentioned in most LD50 test results. The oral LD50 values for linalool range from 2.2 to 3.9 g/kg, which is equivalent to an average adult human drinking 154 – 270 g (5.4 – 9.5 oz). In one of the studies, a non-fatal dose of linalool had a sedative effect on mice when injected into the abdomen at 178 mg/kg, and impaired muscle co-ordination (Atanassova-Shopova et al 1973). This is equivalent to a human dose of 12.5 mL, or 0.44 oz.
This information is entirely derived from LD50 testing of linalool (Jenner et al 1964, Letizia et al 2003). This is the classic test to find the single lethal dose for any substance. Rats and mice are most commonly used, and the dose cited is the one that is lethal to 50% of the animals. When you give a mammal a fatal dose of a substance it is not unusual to see some adverse effects on the nervous system, such as staggering, difficulty breathing etc., nor is it surprising if there are “respiratory disturbances leading to death.” Ataxic (unsteady) gait is probably mentioned in most LD50 test results. The oral LD50 values for linalool range from 2.2 to 3.9 g/kg, which is equivalent to an average adult human drinking 154 – 270 g (5.4 – 9.5 oz). In one of the studies, a non-fatal dose of linalool had a sedative effect on mice when injected into the abdomen at 178 mg/kg, and impaired muscle co-ordination (Atanassova-Shopova et al 1973). This is equivalent to a human dose of 12.5 mL, or 0.44 oz.
None of this means that your dryer sheets are going to kill you or your family. Nor will they cause you to faint, sway, fall over, lose control of your muscles, or otherwise behave as if drunk or dying. If you have multiple chemical sensitivity you may react adversely to any fragrance material, but not necessarily because that substance is itself inherently toxic. Unless you are in the habit of either drinking linalool by the cupful or injecting half an ounce of it into your abdomen, you may safely ignore these dire warnings, which have absolutely no relevance to the use of linalool in cosmetic or household products.
Conclusions
At least as far as essential oils are concerned, the EWG database reveals a shocking degree of ineptitude. They seem to have no idea which essential oils contain which constituents, and they only know about legal restrictions, which they automatically support 100%. If the EU says that linalool is a skin allergen, then it must be right. The EWG staff don’t seem to have read most of the toxicological literature, which they simply give a PubMed link to, and throw this in under “Data gaps”. They are just tossing out information hoping that some of it will stick. There is no science-based risk assessment, and the hazard ratings don’t tell you how much (or how little) of a substance is safe.
The EWG has helped stir up considerable hysteria about cosmetic safety. Some of it may be justified, but their opinion on fragrance is shameful. How can all fragrances present the same level of risk? Increasingly, we see articles, blog posts and videos put out by people who are repeating misinformation and who often have no clue what they are talking about. That this should lead to the targeting of essential oil constituents is ironic, considering the very real healing benefits that the oils have to offer – from skin cancer prevention, to the treatment of antibiotic-resistant infections. And it is happening because of ignorance. We seem to entering a new Dark Age, where truth is measured by Google hits, and scientific fact no longer counts for anything. In some cases safety legislation, instead of reflecting the science, is usurping and replacing it. Another irony is how EU cosmetics legislation is regarded in North America with something approaching reverence while in Europe it is regarded as, at worst, a Nazi-based tyranny (I’m not making this up – there’s quite a conspiracy theory…) and at best, an unecessary hassle – there’s very little evidence that it has had any positive effect at all.
References
Atanassova-Shopova S, Roussinov KS, Boycheva I 1973 On certain central neurotropic effects of lavender essential oil. II communication: studies on the effects of linalool and of terpineol. Bulletin of the Institute of Physiology, Bulgarian Academy of Sciences 15:149-156
De Groot, AC 1987 Contact allergy to cosmetics: causative ingredients. Contact Dermatitis 17:26-34
De Groot AC, Coenraads PJ, Bruynzeel DP et al 2000 Routine patch testing with fragrance chemicals in the Netherlands. Contact Dermatitis 42:184-185.
Fregert S, Hjorth N 1969 Results of standard patch tests with substances abandoned. Contact Dermatitis Newsletter 5:85
Frosch PJ, Pilz B, Andersen KE et al 1995 Patch testing with fragrances: results of a multicenter study of the European Environmental & Contact Dermatitis Research Group with 48 frequently used constituents of perfumes. Contact Dermatitis 33:333-342
Gilpin S, Maibach H 2010 Allergic contact dermatitis from farnesol: clinical relevance. Cutaneous & Ocular Toxicology 29:278-287
Hostýnek JJ, Maibach HI 2003a Is there evidence that anisyl alcohol causes allergic contact dermatitis? Exogenous Dermatology 2:230-233
Hostýnek JJ, Maibach HI 2003b Is there evidence that linalool causes allergic contact dermatitis? Exogenous Dermatology 2:223-229
Itoh M, Ishihara M, Hosono K et al 1986 Results of patch tests conducted between 1978 and 1985 using cosmetic ingredients. Skin Research 28(Suppl.2):110-119
Jenner PM, Hagan EC, Taylor JM et al 1964 Food flavorings and compounds of related structure I. Acute oral toxicity. Food & Cosmetics Toxicology 2:327-343
Letizia CS, Cocchiara J, Lalko J et al 2003 Fragrance material review on linalool. Food & Chemical Toxicology 41:943-964
Santucci B, Cristaudo A, Cannistraci C et al 1987 Contact dermatitis to fragrances. Contact Dermatitis 16:93-95
SCCNFP 1999 Opinion concerning fragrance allergy in consumers: a review of the problem. SCCNFP/0017/98 Final
Schnuch A, Uter W, Geier J et al 2007 Sensitization to 26 fragrances to be labelled according to current European regulation. Results of the IVDK and review of the literature. Contact Dermatitis 57:1-10
Vocanson M, Goujon C, Chabeau G et al 2006 The skin allergenic properties of chemicals may depend on contaminants – evidence from studies on coumarin. International Archives of Allergy & Immunology 140:231-238
Vocanson M, Valeyrie M, Rozières A et al 2007 Lack of evidence for allergenic properties of coumarin in a fragrance allergy mouse model. Contact Dermatitis 57:361-364


Thank you Robert. One comment: You say “That this should lead to the targeting of essential oil constituents is highly ironic, considering the very real healing benefits that they have to offer – from skin cancer prevention, to the treatment of antibiotic-resistant infections.”
Remember that in the US, no one who makes essential oils or products containing them available to the public is allowed to state the healing benefits they offer, lest the appropriate government agency decide that we are selling “untested/unlabeled “New Drugs”.
Thank you for this informed article. A focus of my skin care and perfumery business is informing customers about the contents of products and urging consumers to seek thorough information before submitting to scare tactics. This article is an excellent example of “checking the science” behind the scare tactics!
Thank you Robert for such an informative article. As I read this, I had a striking notion of both “Alice in Wonderland” and “1984”……. down is up, wrong is right!
Thank you Robert for another informative and well-research repsponse to the nonsense from the EWG and the EU! One can grow weary and gray from having to re-educate so many who receive this misinformation on a regular basis from such a group as EWG. I have to say that I am on their list and frequently receive requests for donations to help support their work. I have to wonder then, where does all the money go?
Having attempted, myself, to create a comprehensive article along these lines (and getting bogged down), I KNOW how much diligent effort this was, Robert. Please picture me in supplication, thanking you profusely for giving the rest of us such solid information, so appropriate for fact-supported rebuttal when needed. I was very much disturbed (but sadly not surprised) by the reference under Neurotoxicity – House Committee on Science & Technology, Report 99-827 Sept 16 1986 – which report I wanted to examine myself. If for no other reason than finding reference to it in at least 250+ blogposts and reports using it as a basis, with few if any links to it or other references, many of them simply parroting similar weak articles lacking credible supportive evidence. This puts a new slant on the word “dittohead”, or at least expands the definition. 🙂 In the past (before pseudo science came to play such a large roll in politics) one could usually count on credible science in most government reports. Those days appear to be long gone, and unless we can find a copy of that actual report, perhaps since 1986 or even earlier. I did find what were supposed excerpts from the report, but I’m still looking for the report itself. Affirming, once again, that credible journalistic referencing is as bad, if not worse than credible scientific referencing in the modern era of political spin. And, make no mistake, much of this is political spin funded by those who benefit. I think we have a long road ahead to secure a resurrected place for rational scientific documentation, soundly based in rigorous academia. You’ll find a cadre of politicians and think tanks polluting the media outlets and cyberspace with their version of “not to worry” while knowing they are harming us by withholding the real science that might cost their corporate benefactors to clean up their act with regard to environmental pollution. And, we who are aromatherapists or natural health care providers find ourselves on both sides of the coin, having to defend credible science while disputing the pseudo variety. So, I can relate to both of Gina’s notions above. And, may I add, in the words of the cartoon Cathy – ACK!!
Thank you Marge, Katie, Gina, Lora and Marcia. And I know you can imagine, Marcia, the many hours – over many weeks – that it took to put this all together. And yes, here we are in the “information age” where tech gurus are still working hard to bring us to the point where we all have access to all the information there is about everything! I’m delighted that I can, by clicking my mouse, access a great many scientific papers. But one downside is that everyone’s an “armchair toxicologist” now. And, misinformation spreads just as fast as information. In many ways we are in fact in the misinformation age.
Five dermatitis patients having allergic reactions over 5 years of concentrated patch testing is quite a standard — I wonder if all other ingredients would pass that bar.
Fantastic piece of work Robert, I am so grateful to you for having taken the time and energy to have done this research for us all. There is no doubt about the many hours you have put into this article, and the information you have shared should be a great source of education for many.
Thank you!!! Aromatic blessings!
Thank you so much for this paper, Robert. Your breadth and depth of knowledge, coupled with your ability to focus on the real problem, not the imagined one, is always a beacon of hope. I am looking forward to your presentation here in Miami in October, and I am very glad you are on the lecture circuit, spreading common sense where fairy tales abound. One day all this effort on your part will be recognized, and hopefully, the regulatory forces will reorient themselves.
A great paper for our international students, well worth all the hard work. I agree folk in North America shouldnt interpret EU Legislation out of its local context. There is the political integrative agenda to get the EU brand onto every label however small! Correctly farmed and distilled essential oils are viewed as very much part of the solution here at least by the public to judge by the demand for essential oils. Thanks to your efforts we know what matters in the aromatherapy world crucially is the date of distillation and informed and trained use in correct low concentrations of true whole oils.
As usual with well intentioned regulation what actually matters is in danger of being swamped in a mass of irrelevant and harmless detail. Sight must not be lost of what and who set this in motion which was and is irresponsible fragrancing of everything from detergent to nappies with ‘lavender’ or ‘lemon’ which is then left in the home environment to oxidise and sensitise. It is this pursuit of $$$ heedless of the consumer that the EU is trying to address in its own way and not to stigmatise responsible essential oil farmers and aromatherapists.
So where does this leave us today? As to blurb the Sp box of Rose Otto ingredients now reads “Rosa Damascena oil, Citral, Citronellol, Eugenol, Farnesol, Geraniol, Limonene, Linalool”. When we do the next print of labels we are going to put the word ROSE OTTO into very very large letters to remind us whats actually in the bottle! Similarly the Jasmine box ingredients reads “Jasminum Officinale oil, Benzyl alcohol, Benzyl Benzoate, Eugenol, Linalool. No its JASMINE!
It’s good to hear from you Ian, though I don’t understand what you mean by “local context”, and I don’t think I suggested anything like that! Why should linalool or benzyl alcohol be any more or less safe in one region than in another? You seem to be suggesting that the EU legislation is there to protect consumers. From fragranced diapers? What irrelevant and harmless detail? I’m pretty confused…
Thank you so much Robert for this great post…looking at the data i really don’t know where and why IFRA goes so though on allergens labelling…and the big problem is that the majority of people are covered by disinformation…EU is a mess…no protection, just complicating life.
greetings
Hi Robert see you the Miami seminar. Looking forward to it. By local context I mean the EU are just saying that if you happen to be one of the very few who are sensitive to an allergen for example to linalool then you should know that certain of these products contain linalool. Just because a few people are allergic to gluten wheat is not banned or we’d starve! EWG should not read more into it than that. Natural products are very much part of the solution. Unfortunately the tendancy for regulators is to wallow in irrelevant and harmless detail. For example in the realm of financial services the regulators in the UK in the 1990s started to follow micro investment advice however harmless or trivial and yet completely missed or turned a blind eye to wild greedy behaviour in banking which cost hundreds of thousands if not millions of jobs. A regulatory system is a machine. Can a machine be wise?
I am printing this article out to re-read…maybe a few more times. Thanks so much for the pertinent information that really clarifies the issues we face using essential oils and the misinformation by the regulating – type organizations.
Ragna
I have just one word for this Article and Effort: EXQUISITE!
Thank you so much for writing this article, Robert!
In considering how best to kill with a dryer sheet, you have made me aware that the best way is to string them together as a rope and not to wait for the toxic effects of Linalool. Thank you Robert for your continued excellent work and salient thoughts.
Extremely informative article – very well written.
Thanks Robert,
useful stuff to quote when so many are trying to restrict the use of natural products, (largely in my view to allow their chemically produced products with a multinational behind them to increase or maintain sales.)
I am reminded that I have seen Juniper falsely listed as an abortificant and contraindicated in pregnancy when the only evidence of this that I know of is that it is used in Gin which was associated with ilegal abortions. Obviously the gin was merely used as a cheap source of alcohol and the fact that it contained Juniper was irrelevant.
Much more on your blog for me to read about.
Dave.