Special thanks to Ken Harper of tendigitgifts.com for making this interview available in Spanish: Este entrevista en Español!
Kevin M Dunn has a PhD in Chemical Physics and is currently the Elliott Professor of Chemistry at Hampden-Sydney College, Virginia. His book, Caveman Chemistry, brought him to the attention of soapmakers and he subsequently directed a series of research projects on the chemistry of handcrafted soap. This culminated in a second book, Scientific Soapmaking, which was published in 2010. More information here.

Kevin Dunn
It was a pleasure to meet Kevin Dunn in Miami at the 2011 Handcrafted Soapmaker’s Guild Conference, where we were both giving presentations.
Robert
Could you briefly explain the main processes used to make handcrafted bar soap?
Kevin
The handcrafted soap business is divided into two main segments: MP (Melt and Pour), and CP/HP (Cold Process/Hot Process). Melt and Pour soap base is already soap when the soapmaker buys it. The soapmaker melts the soap base, adds scents and colors, and pours it into a mold. In this process, scents (including essential oils) remain intact for the most part.
CP/HP soap starts out as a mixture of fats, oils, and lye. In HP soap, the raw soap is cooked until the lye has been consumed, after which, scents and colors may be added. In CP soap, however, scents and colors are generally added before the lye has been consumed, while the raw soap is highly alkaline. Scents and colors cannot be added to CP soap after the lye has been consumed because by this point the soap is solid. For this reason, CP soapmakers must either choose scents and colors that are resistant to strong alkali, or accommodate themselves to the changes that occur in non-resistant scents and colors.
Robert
Is mass-produced bar soap made using different processes?
Kevin
Commodity soap is produced by several processes, but they all share the property that the soap is made from fats and oils, shredded, mixed with scents and colors, and then pressed into bars. In this respect, it is more like MP or HP soap—scents and colors are not subjected to harsh alkaline conditions because they are not added until after the alkali has been consumed.

Making liquid soap
Robert
What about liquid soap?
Kevin
Commodity liquid “soap” is most often a detergent. Handcrafted liquid soap is a true soap produced by a Hot Process. Because the soap remains liquid, however, scents and colors may be added after the alkali has been consumed.
Robert
Which processes and which essential oils are most likely to lead to evaporation of essential oil constituents, and how can this be minimized?
Kevin
An essential oil with a low boiling point may suffer loss in a MP or HP soap, and this information may be found in the MSDS for the oil. Look to ensure that the temperature of an MP or HP soap is lower than the boiling points of your essential oils when they are added.
Robert
Discussions among soap makers often touch on whether or not EO constituents saponify. Is there a simple answer to this?
Kevin
There is an answer, but it is not simple. Essential oils are complex mixtures of dozens of chemical compounds. A given essential oil may contain some compounds that react with alkali, and others that do not. Lavender oil, for example, contains about 42% linalool (which does not react) and 22% linalyl acetate (which does). In fact, when linalyl acetate reacts with alkali, one of the products is linalool. Thus the scent of a CP soap made with lavender oil will smell less of linalyl acetate and more of linalool than the original EO.
The only way to predict which essential oils will react with alkali is to examine the list of components and note which of them are reactive. Such compounds generally consist of esters, phenols, and acids. There is a practical way, however, for a soapmaker to evaluate essential oil reactivity. Add a few drops of essential oil to 1 mL of the lye solution used for soapmaking (typically 25%-50% NaOH). Sometimes a reaction will be visible and sometimes not. In either case, wait a day or two and then compare the scent of the alkaline EO to that of the original. In some cases, there will be no difference in scent. In those cases where the scent changes, the alkaline scent might not be bad, just different from the original.

Robert
Specifically, what happens to esters, phenols and acids present in EOs when they are used in soap?
Kevin
Phenols and acids react directly with alkali to produce odorless salts. Clove oil for example, contains a large proportion of eugenol (a phenol). If you add a few drops of clove oil to lye as described above, the resulting solution is bright yellow and very nearly odorless. Esters, on the other hand, are decomposed by lye into an acid salt (usually odorless) and an alcohol, which is often fragrant. In fact, the alcohol produced is often present as one of the components of the original EO. The scent of such an EO changes as the proportions of its components change, but it remains fragrant.
Robert
Are any other EO constituents affected by soap making processes?
Kevin
Not that I am aware of. The name of an ester is easy to spot: something-yl something-else-ate (e.g. linalyl acetate, methyl salicylate, methyl benzoate). Phenols are harder to spot, but the most common fragrant phenols are eugenol (clove, cinnamon leaf), carvacrol (thyme, oregano), thymol (thyme), and vanillin (vanilla). Phenols are actually weak acids. Other fragrant acids typically smell sour, e.g. acetic acid in vinegar.
Robert
Thank you Kevin, this is really useful information!
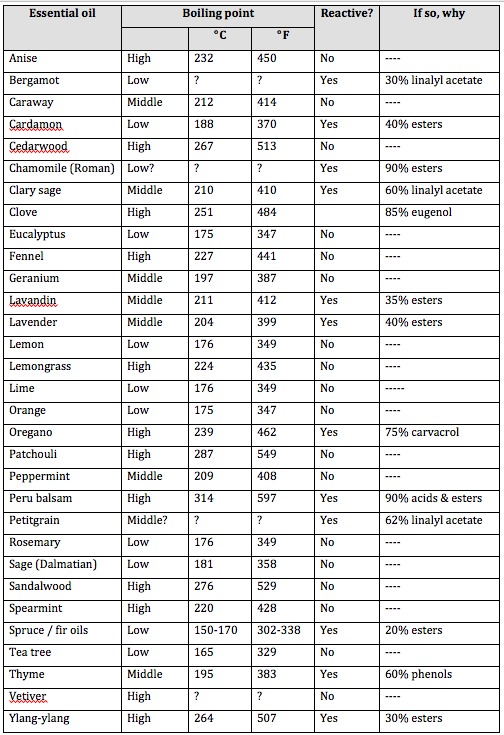
The Table below is a rough guide to the boiling points of some commonly-used oils, and whether or not they are likely to be chemically changed through contact with alaki. Boiling points were taken from online sources, and I cannot vouch for accuracy. In some cases, I could not find any information on boiling points. The constituent information is mine. It is representative of a typical essential oil, but there is always natural variation. Don’t confuse Low, Middle, High with the Top, Middle and Base classification used in perfumery! In this Table, “High” means high boiling point, which correlates with low evaporation rate. Conversely, a “Low” boiling point means that it evaporates readily – a “top” note in perfumery. “Reactive?” means “is the essential oil likely to react with alkali”. The greater the percentage of acids esters or phenols, the more of the oil will be changed. But remember, the oil may still be fragrant, and actually it may still be therapeutic too. (Note that very few essential oils contain significant amounts of carboxylic acids, and these are weak acids which are not corrosive.)


Great information Robert — just what the handcrafted soapmaking industry has needed.
This is an excellent post! Since I use only essential oils in all of my products, I found the reaction of EO’s in soap making to be very beneficial. It’s nice to get the scientific perspective on what chemically happens to the EO in the CP process. I am so glad the two of you colaborated on this piece. Well done indeed!!!
What an incredibly informative article! I don’t make soap and I read every word because it was fascinating. Robert, you need a “share” button to Facebook and Twitter for your blog articles, it really helps spread the word.
What a fantastically educational post! Thank you Robert.
hello. i use EO in my soaps. this note seem to be very interesting!!!
haven´t you an spanish traslation? please!!!
I enjoyed reading this post. I recently made a cold process soap with 3 different types of lavender essential oil and the scent came through nicely. As a formulator, I am fascinated how the scent from essential oils will “turn” in acidic or akali products like lotions and creams. Thank you, this information will assist in formulating.
Thank you for the information. You both have contributed much to the Handcrafted Soapmaker’s community.
What an informative conversation. Thanks so much for the valuable info. I’m glad I subscribed to the newsletter.
Thanks Robert! Kevin has really brought soap making a long way. I love his books, articles, and anything else I can find.
Lara, here’s your translation; In the future, you can use Google Translate to translate any text….
English to Spanish translation
Los aceites esenciales de jabón: entrevista con Kevin Dunn
Kevin M Dunn tiene un doctorado en Química Física y actualmente es el profesor Elliott de Química de Hampden-Sydney College, Virginia. Su libro, Química hombre de las cavernas, lo trajo a la atención de los fabricantes de jabón y posteriormente dirigió una serie de proyectos de investigación sobre la química del jabón artesanal. Esto culminó en un segundo libro, Fabricación Científico de Jabón, el cual fue publicado en 2010. Más información aquí.
Para mi, fue un placer conocer a Kevin Dunn en Miami en la Conferencia de Hacer Jabón Artesanal 2011, donde estábamos dando presentaciones los dos.
Robert
¿Podrías explicar brevemente los procesos principales utilizados para hacer jabón artesanal?
Kevin
El negocio de jabón artesanal se divide en dos segmentos principales: MP (Derrita y vierta), y la CP / HP (en frío / caliente de proceso). Derrita y vierta base de jabón es jabón ya que el fabricante de jabón que compre. El fabricante de jabón se derrite la base de jabón, añade aromas y colores, y lo vierte en un molde. En este proceso, los olores (incluyendo aceites esenciales) permanecen intactos en su mayor parte.
CP / HP jabón que comienza como una mezcla de grasas, aceites y la lejía. En HP jabón, el jabón crudo se cocina hasta que la lejía se ha consumido, después de lo cual, aromas y colores que se pueden añadir. En jabón CP, sin embargo, los olores y los colores son por lo general antes de añadir la lejía se ha consumido, mientras que el jabón crudo es altamente alcalino. Los olores y los colores no se pueden agregar a CP jabón después de la lejía se ha consumido ya por este punto, el jabón es sólido. Por esta razón, fabricantes de jabón CP pueden pagar los olores y colores que son resistentes a los álcalis fuertes, o acomodarse a los cambios que se producen en los no resistentes a los olores y colores.
Robert
Es producido en serie una barra de jabón hecho con los diferentes procesos?
Kevin
Materias primas de jabón se produce por varios procesos, pero todos ellos comparten la propiedad de que se hace el jabón de las grasas y aceites, triturados, mezclados con aromas y colores, a continuación, pulsa en barras. En este sentido, se parece más a MP y HP jabón aromas y colores que no son sometidas a duras condiciones alcalinas, ya que no se añaden hasta que el álcali se ha consumido.
Robert
¿Qué pasa con el jabón líquido?
Kevin
Materias primas líquidas “jabón” es a menudo un detergente. Jabón líquido hecho a mano es un jabón verdadero producido por un proceso en caliente. Debido a que el restos de jabón líquido, sin embargo, aromas y colores que se pueden añadir después el álcali se ha consumido.
Robert
Que los procesos y que los aceites esenciales son más propensos a conducir a la evaporación de los componentes del aceite esencial, y cómo puede reducirse al mínimo?
Kevin
Un aceite esencial con un bajo punto de ebullición pueden sufrir la pérdida de un diputado o un jabón de HP, y esta información se puede encontrar en la ficha de seguridad para el aceite. Mire para asegurarse de que la temperatura de un jabón MP o HP es inferior al punto de ebullición de los aceites esenciales que se añaden.
Robert
Las discusiones entre los fabricantes de jabón suelen tocar o no componentes AE saponificar. ¿Hay una respuesta simple a esto?
Kevin
Hay una respuesta, pero no es simple. Los aceites esenciales son mezclas complejas de decenas de compuestos químicos. Un aceite esencial dado pueden contener algunos compuestos que reaccionan con el álcali, y otros que no. El aceite de lavanda, por ejemplo, contiene alrededor del 42% de linalol (que no reacciona) y el 22% acetato de linalilo (que sí). De hecho, cuando reacciona con el acetato de linalilo alcalinos, uno de los productos es el linalol. Así, el olor de un jabón CP hecho con aceite de lavanda se huele menos de acetato de linalilo y más de linalol que el AE original.
La única manera de predecir que los aceites esenciales van a reaccionar con álcali es examinar la lista de componentes y tenga en cuenta que de ellos se reactiva. Estos compuestos generalmente consisten en ésteres, fenoles y ácidos. No hay una manera práctica, sin embargo, para un fabricante de jabones para evaluar la reactividad de aceites esenciales. Añadir unas gotas de aceite esencial en 1 ml de la solución de sosa usado para hacer jabón (típicamente 25% -50% de NaOH). A veces una reacción serán visibles y otras no. En cualquier caso, esperar un día o dos y luego comparar el olor de la EO alcalina a la del original. En algunos casos, no habrá diferencia en el olor. En aquellos casos en que los cambios olor, el olor alcalino puede no ser malo, sólo diferente de la original.
Robert
En concreto, lo que sucede a los ésteres, fenoles y los ácidos presentes en organizaciones de empleadores cuando se usan en el jabón?
Kevin
Fenoles y los ácidos reaccionan directamente con el álcali para producir sales de olor. El aceite de clavo por ejemplo, contiene una gran proporción de eugenol (un fenol). Si se añaden unas gotas de aceite de clavo de la lejía como se describió anteriormente, la solución resultante es de color amarillo brillante y muy cerca del inodoro. Ésteres, por el contrario, se descomponen con lejía en una sal de ácido (generalmente sin olor) y un alcohol, que es a menudo fragantes. De hecho, el alcohol producido a menudo se presenta como uno de los componentes de la AE original. El aroma de este tipo de AE cambios como las proporciones de los componentes de su cambio, pero sigue siendo fragante.
Robert
¿Hay otros componentes AE afectados por los procesos de fabricación de jabón?
Kevin
No es que yo sepa. El nombre de un éster es fácil de identificar: algo-il-algo más-ate (por ejemplo, acetato de linalilo, salicilato de metilo, benzoato de metilo). Los fenoles son más difíciles de detectar, pero los fenoles aromáticos más comunes son el eugenol (clavo, canela, hojas), carvacrol (tomillo, orégano), timol (tomillo), y la vainilla (vainilla). Los fenoles son realmente ácidos débiles. Otros ácidos aromáticos suelen oler por ejemplo, ácido, ácido acético en el vinagre.
Robert
Gracias Kevin, esto es realmente información útil!
La tabla siguiente es una guía aproximada de los puntos de ebullición de algunos aceites de uso común, y si es probable que sea alterada químicamente a través del contacto con alaki. Puntos de ebullición se tomaron de fuentes en línea, y no puedo dar fe de la exactitud. En algunos casos, no pude encontrar ninguna información sobre los puntos de ebullición. La información constituyente es la mía. Es representante de un aceite esencial típica, pero siempre hay variación natural. No confunda Bajo, Medio, Alto con el Superior, Medio y la clasificación de base utilizadas en perfumería! En este cuadro, “High” es alto punto de ebullición, que se correlaciona con la velocidad de evaporación baja. Por el contrario, un “bajo” el punto de ebullición significa que se evapora con facilidad – un “top” nota en la perfumería. “Reactiva?” Significa “es el aceite esencial propensos a reaccionar con álcali”. Cuanto mayor es el porcentaje de ésteres de ácidos o fenoles, más del petróleo será cambiado. Pero recuerde, el petróleo todavía puede ser aromático, y de hecho todavía puede ser terapéutico también. (Tenga en cuenta que los aceites esenciales muy pocos contienen cantidades significativas de ácidos carboxílicos, y estos son ácidos débiles que no son corrosivos.)
Robert, This is an excellent source of information and I so thank you for sharing this with us soapmakers. Now I have more direction in what to use for M&P scenting and my HP scenting. Also, a heads up on what is reactive really makes a big difference!
Thank you so much!
Thank……………… you so much for the valuable info.
Robert, that was superb information ! Helps me to market our Essential Oils to Soap Makers.
An excellent and very useful article, thank you
Very informative article. Thank you. Now I know what Mr Dunn looks like.
Anya, share button now installed for social media!
Robert and Kevin, this was fun to read and informative! I especially appreciate the chart! Thank you.
Thank you, such thorough information! Always looking for more knowledge on the things I formulate with. And the share button–much appreciated!
Robert,
Thank you so kindly for this invaluable information. As a fairly new soapmaker it will indeed be of great assistance. Thank you both for sharing your knowledge and expertise. Brilliance!
Ashley
Thank you Robert and Kevin. This will be shared with many soapmakers!
Can we make any assumptions on safe levels of essential oils in CP or HP soap compared to melt and pour? I’m trying to make sure I’m using essential oils safely in my CP soap. I recently have been pondering if safe levels listed by IFRA on wash of products are for commercial soaps and not CP soap or if there is a difference! I’m so happy I found your site!
Lori – well as far as IFRA is concerned, there is no difference between one soap-making process and another, all “wash products” are treated equally. Obviously it’s the amount left in the soap at the end that counts, not necessarily the amount you add, but I don’t know if there’s any way to accurately estimate fragrance loss through evaporation, and anyway it’s only going to be a small % that’s lost. So anyway, I would say there’s no difference!
Hello, great interview thank you. I would like to ask if there has been a study done to see if any of the therapeutic properties of essential oil remain intact through the process of saponification. Do the properties change in any way through the process considering they are exposed to high heat as well as the chemical transformation taking place as the soap saponifies?
Thanks in advance….
Hi Linda. I am not aware of any peer-reviewed studies that directly compare soap without essential oil to the same soap with essential oil, in terms of antibacterial or any other properties. But, there is plenty of anecdotal evidence that essential oils in soap are active. The heat may cause some loss of essential oil molecules through evaporation, but it should not cause any significant chemical changes. The contact with alkali will cause some chemical changes, and of course that’s what Kevin describes in the interview. These changes may or may not result in an alteration of therapeutic properties. That depends on which properties you are measuring and also on which constituents you are talking about, so there isn’t a simple answer. Except perhaps to say that in MOST instances essential oils do in fact retain their therapeutic action!
What a fantastic interview! Thank you so much for the information – the chart is just brilliant. I’ve printed and added it to my soap notebook. I wish I had been able to see you speak at the last HSMG convention, but hopefully there will be other opportunities. 🙂 Thanks again!
I must add my kudos for a great article, very informative. To clarify a point, is it true that when the boiling point of an eo is low (meaning it will easily evaporate), more of that eo must be added to a blend compared to another eo in the blend with a higher boiling point, in order to keep more of a balance between the two scents?
Thank you so much for this information, Robert. Words cannot express my gratitude. I have been searching for nearly three years for information on this subject — with little to no success. The fragments I have found, I did not trust. You and Kevin Dunn are two individuals that I trust very highly. As Val said above, the chart is extremely valuable. I hope that more research will be done regarding using essential oils to create “real” soap that is antibacterial.
This information is very interesting, and highly benificial for soap makers. Thanks, Danielle Sade
Thank you very much, for this invaluable informations, especially for the chart..
thank you for the informations..
Fantastic information! Thank you so much, Kevin and Robert. I’ve learned how essential oils are affected by saponification mostly through trial-and-error over 15 years of soapmaking; it is so helpful to read about the chemistry behind these changes. The chart is an especially invaluable reference. Thank you!
This is the most pertinent information on essential oils and their action on soap that I have found….unfortunately, I found it after I wrote my book and not before. This will be saved for future reference. Sincere thanks for this post, nothing like this has been discussed that I have found other than the book “Sanitary Products” (Schwartz, 1943), which concurs with the information Kevin provided. Bravo for addressing this issue with a highly esteemed chemist in the handcrafted soapmaking field!
Great article. Thank you for posting again!
Thank you Robert and Kevin for this fantastic and informative article!!! As a CP/HP soap maker I have searched and searched for this very information to no avail. You have made it simple and to the point and I am so grateful!!!
A wonderful and helpful post! Thank you so much for sharing this with us.
Thank you Robert and Kevin for this information! As an aromatherapist who makes CP soap, I scent with essential oils exclusively. Intuition tells me the therapeutic properties persist through the lye process but I have never seen anything written that would validate that hunch, so I particularly appreciate Robert’s response to Linda’s question about that. Thanks all, this is enormously helpful!